Non Muscle Invasive Bladder Cancer
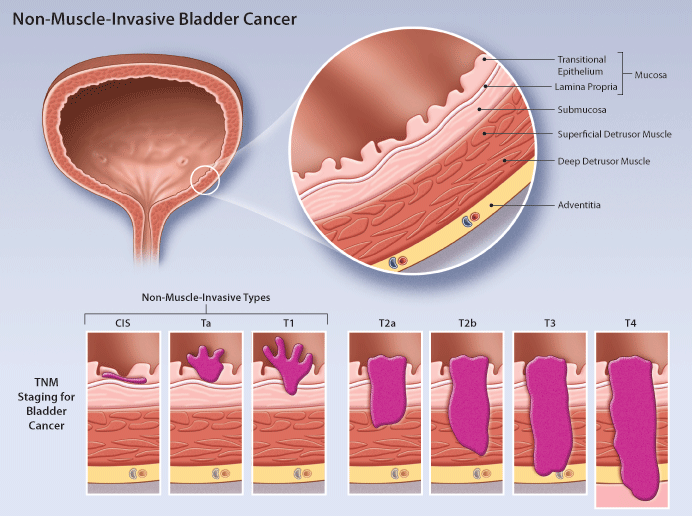
Non Muscle Invasive Bladder Cancer Review Of Diagnosis And Management This guideline provides a risk stratified clinical framework for the diagnosis and treatment of non muscle invasive bladder cancer (nmibc). it covers topics such as cystoscopy, resection, imaging, risk stratification, and variant histologies. Learn about nmibc, a type of bladder cancer that hasn’t reached the muscle layer yet. find out the symptoms, risk factors, diagnosis, and treatment options for this condition.
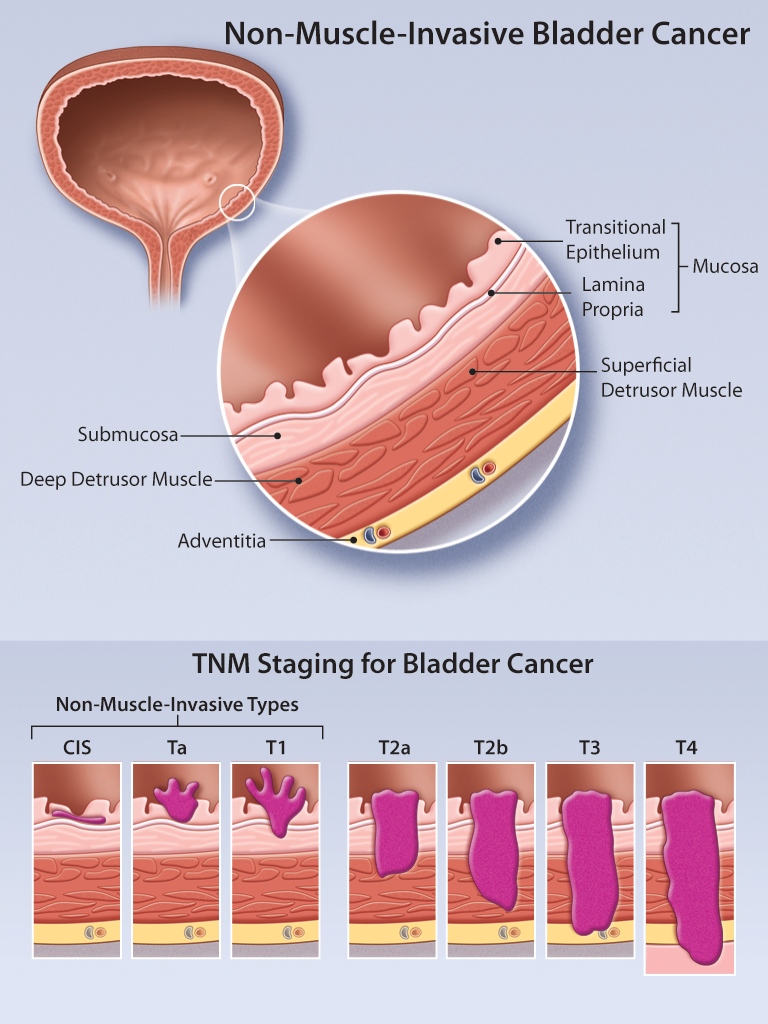
Non Muscle Invasive Bladder Cancer Review Of Diagnosis And Management For non muscle invasive bladder cancer, we generally treat with intravesicle therapy. but there are side effects of treatment: burning pain with urination, frequency, urgency, blood in the urine. they also involve catheterizations and can be painful during administrations. Approximately 70 percent of all new cases of bladder cancer are classified as non muscle invasive, also called superficial bladder cancer. the initial treatment for this stage of bladder cancer is surgical removal of the tumor through a cystoscope, called transurethral resection of bladder tumor (turbt). Non muscle invasive bladder cancer is a diverse subclassification of urothelial bladder cancer with a wide variation in recurrence and progression rates. risk adapted treatment is essential to balance adverse effects of treatment with adequate disease control and there are many nomograms and strategies to assist, each with specific limitations. Treating non muscle invasive (stage 0 and stage i) bladder cancers. non muscle invasive bladder cancers (nmibcs) have not yet grown deep enough to reach the muscle layer of the bladder wall. they include: stage 0a: non invasive papillary carcinoma (ta) stage 0is: non invasive flat carcinoma (tis), also known as carcinoma in situ (cis).
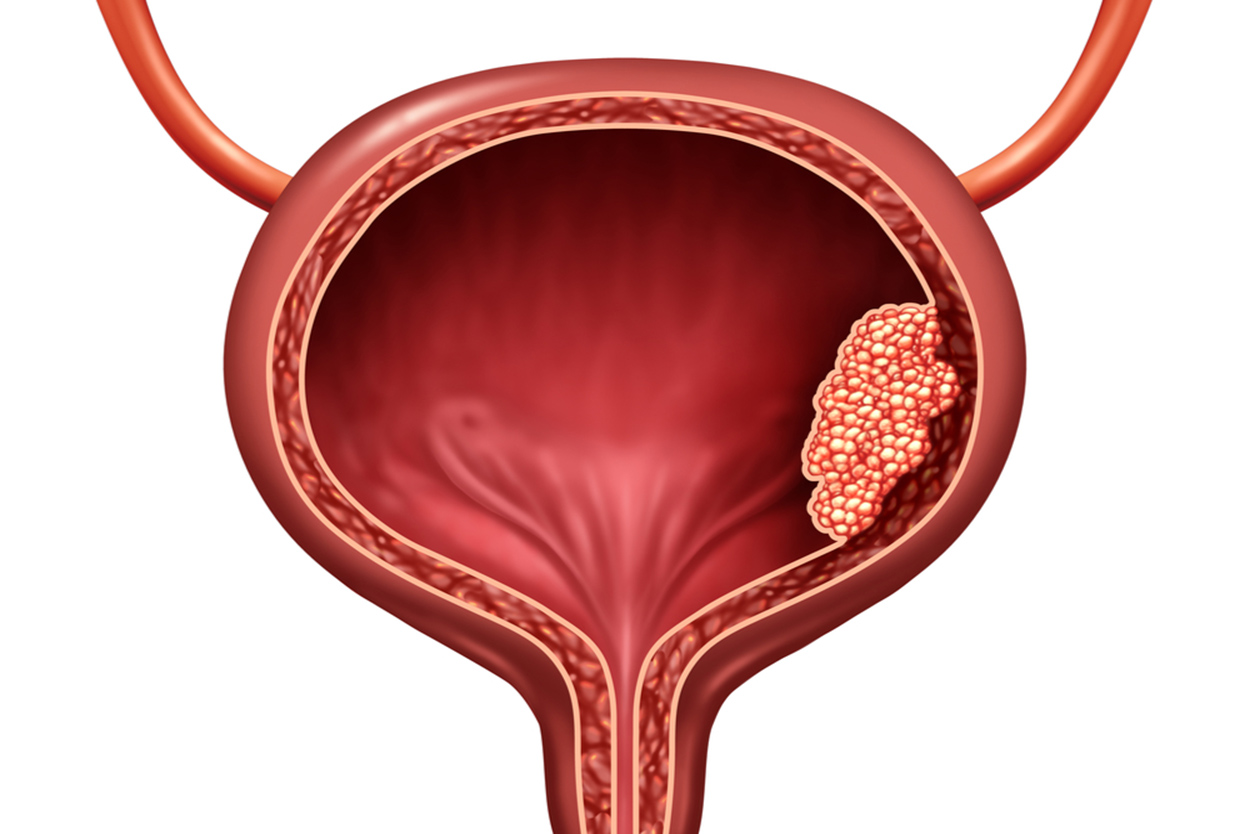
Intravesical Gene Therapy For Non Muscle Invasive Bladder Cancer Ace Non muscle invasive bladder cancer is a diverse subclassification of urothelial bladder cancer with a wide variation in recurrence and progression rates. risk adapted treatment is essential to balance adverse effects of treatment with adequate disease control and there are many nomograms and strategies to assist, each with specific limitations. Treating non muscle invasive (stage 0 and stage i) bladder cancers. non muscle invasive bladder cancers (nmibcs) have not yet grown deep enough to reach the muscle layer of the bladder wall. they include: stage 0a: non invasive papillary carcinoma (ta) stage 0is: non invasive flat carcinoma (tis), also known as carcinoma in situ (cis). High risk nonmuscle invasive bladder cancer. the majority of newly diagnosed bladder cancers (75% to 80%) are classified as nonmuscle invasive bladder cancer (nmibc). treatment for nmibc often includes intravesical therapies. in particular, for patients with high risk nmibc, including those with carcinoma in situ, high grade t1, or large volume. The european association of urology (eau) provides practical recommendations on the clinical management of nmibc, including tat1 and cis. the guidelines are updated annually based on the latest evidence and expert consensus.
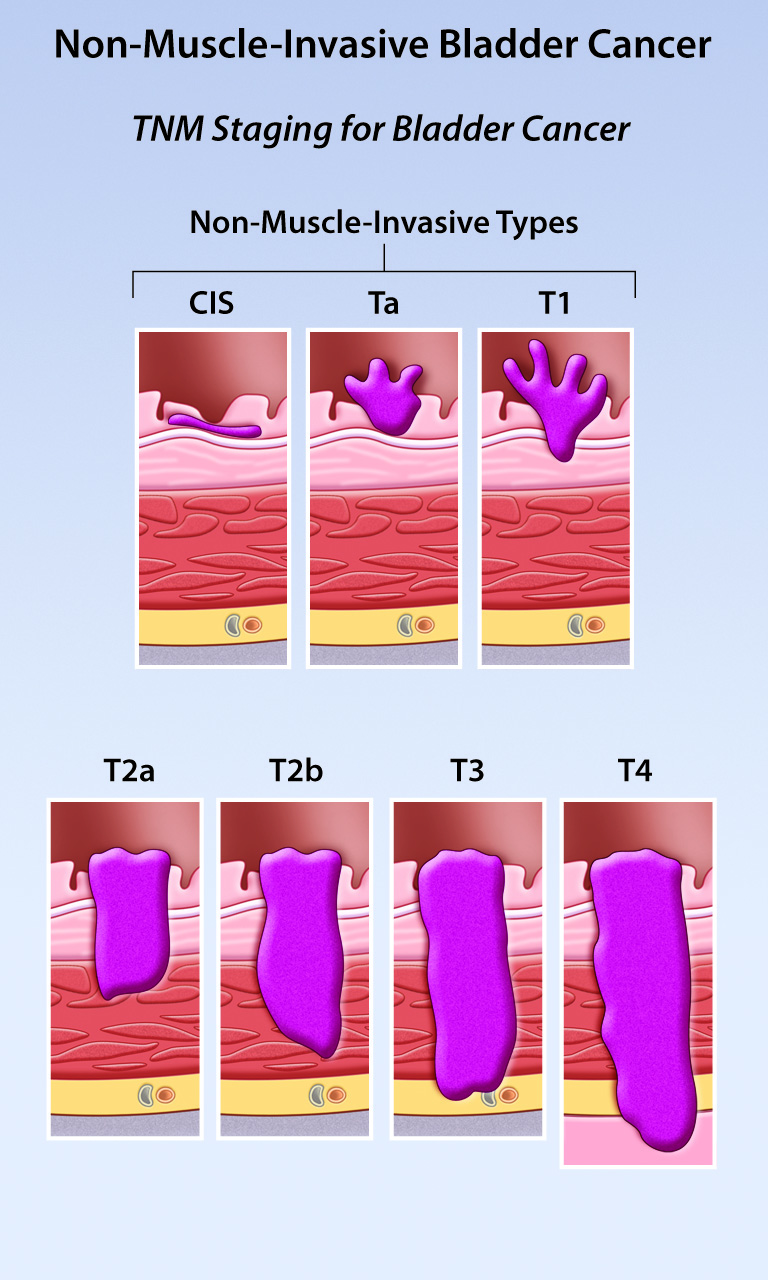
Non Muscle Invasive Bladder Cancer Review Of Diagnosis And Management High risk nonmuscle invasive bladder cancer. the majority of newly diagnosed bladder cancers (75% to 80%) are classified as nonmuscle invasive bladder cancer (nmibc). treatment for nmibc often includes intravesical therapies. in particular, for patients with high risk nmibc, including those with carcinoma in situ, high grade t1, or large volume. The european association of urology (eau) provides practical recommendations on the clinical management of nmibc, including tat1 and cis. the guidelines are updated annually based on the latest evidence and expert consensus.

Comments are closed.