Protein Structure And Folding
Protein Folding Gamma Amino acid structure. amino acids are the monomers that make up proteins. each amino acid has the same core structure, which consists of a central carbon atom, also known as the alpha (α) carbon, bonded to an amino group (nh2), a carboxyl group (cooh), and a hydrogen atom. every amino acid also has another atom or group of atoms bonded to the. Chapter 6 protein structure & folding 3 that many proteins gave a periodic diffraction pattern, suggesting a regular feature in their structures, but the best crystallographers of the day, including william lawrence bragg, were unable to explain the pattern. pauling thought that simple polymers might provide insight into how peptides fold.
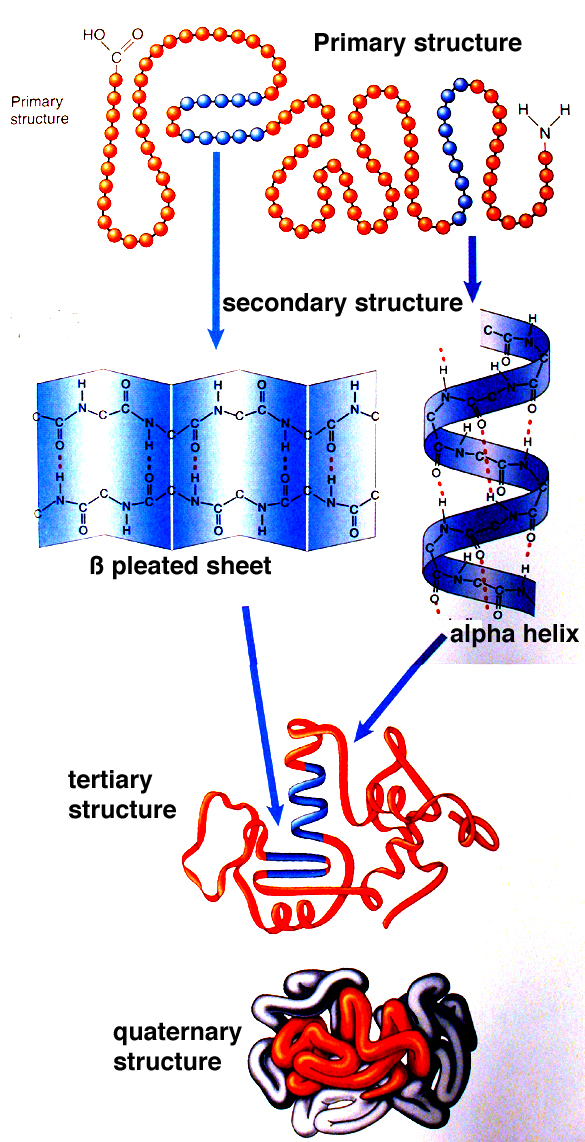
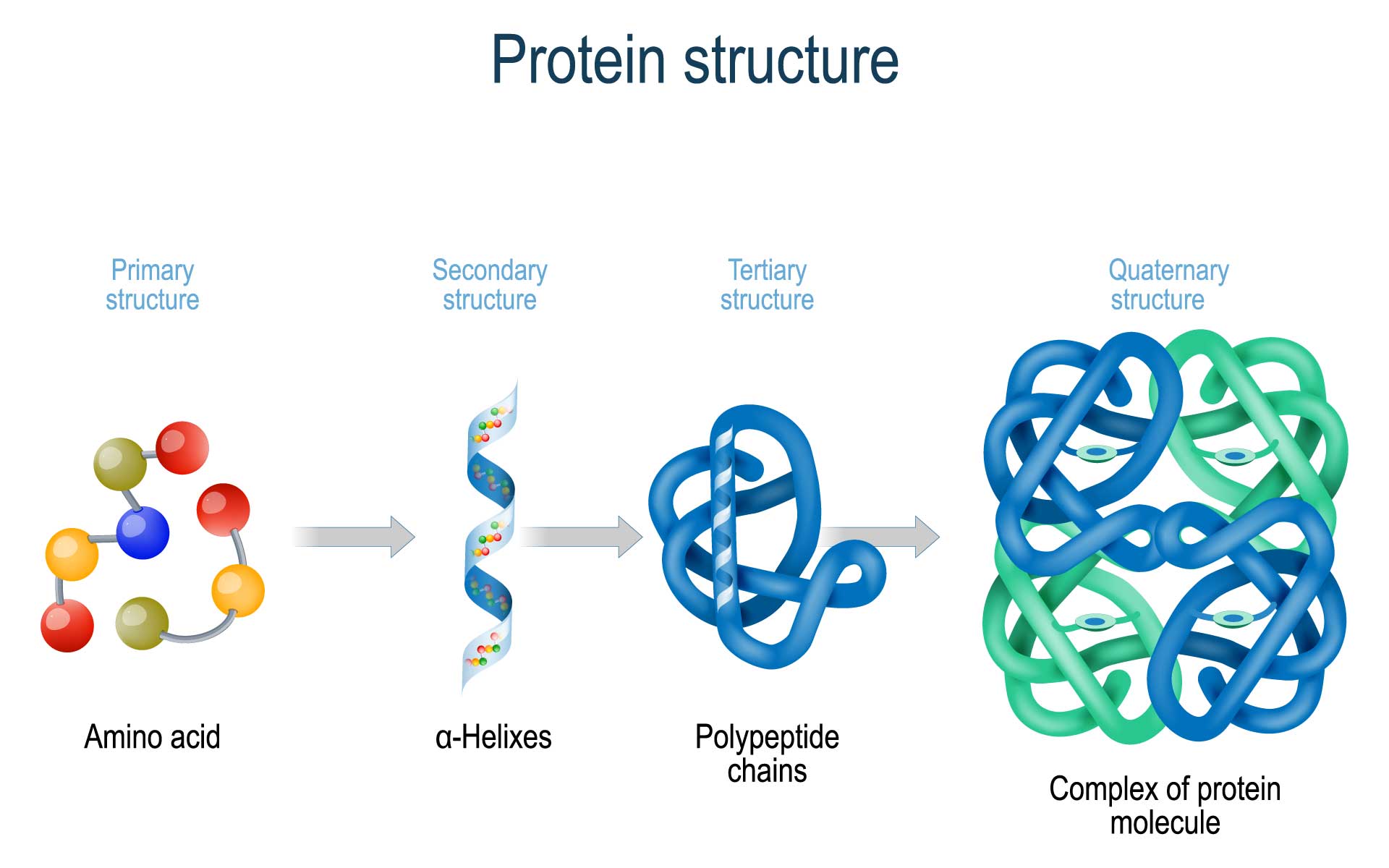
Protein Folding In The Neuron And The Mind Jon Lieff M D The tertiary structure of a protein can often be divided into domains—i.e., distinct compact folding units usually comprising 100 to 200 residues. small proteins may contain a single domain, whereas larger proteins often contain multiple domains. The folding transition and the functional transitions between useful states are encoded in the linear sequence of amino acids, and a long term goal of structural biology is to be able to predict both the structure and function of molecules from the information in the sequence. 6 the subunit organization is the last level of structure in protein molecules. 1 the organization of the subunits is. Protein folding. protein folding is the physical process by which a protein, after synthesis by a ribosome as a linear chain of amino acids, changes from an unstable random coil into a more ordered three dimensional structure. this structure permits the protein to become biologically functional. Learn how proteins are made of amino acids and how they fold into different shapes and conformations. explore the methods and challenges of studying protein structures and the role of chaperone proteins in folding.

Proteins Microbiology Protein folding. protein folding is the physical process by which a protein, after synthesis by a ribosome as a linear chain of amino acids, changes from an unstable random coil into a more ordered three dimensional structure. this structure permits the protein to become biologically functional. Learn how proteins are made of amino acids and how they fold into different shapes and conformations. explore the methods and challenges of studying protein structures and the role of chaperone proteins in folding. The folding is driven by the non specific hydrophobic interactions, the burial of hydrophobic residues from water, but the structure is stable only when the parts of a protein domain are locked into place by specific tertiary interactions, such as salt bridges, hydrogen bonds, and the tight packing of side chains and disulfide bonds. In studying protein folding and stability structure of the native and denatured states, both equilibrium (thermodynamic) and timed (kinetic) measurements are made. folding occurs in the ms to second range, which limits the ability to study the presence of intermediates in the process. some clever methods have been developed to study.

Comments are closed.